Traditionally, identifying new drug candidates has been a lengthy and expensive endeavor, often requiring researchers to sift through vast libraries of molecules to isolate a few promising compounds.
AI is now changing that approach. By leveraging advanced computational techniques, it can design novel molecules and predict their interactions with the human body well before any lab testing begins. This not only enhances the accuracy of early-stage discovery but also reduces the time and investment needed to bring new treatments to life. As a result, pharmaceutical companies are increasingly embracing generative AI to drive innovation and address complex health challenges more effectively.
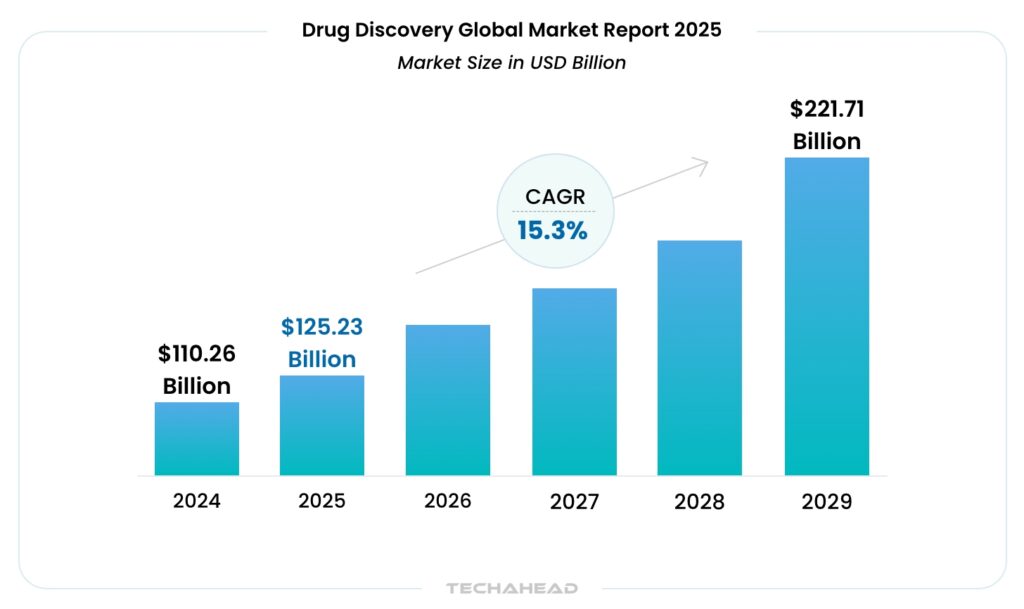
The momentum is also reflected in the market’s growth. The global drug discovery market is expanding rapidly, projected to rise from $110.26 billion in 2024 to $125.23 billion in 2025 alone.
In this blog, we’ll dive into how generative AI is revolutionizing the drug discovery pipeline, unlocking new therapeutic opportunities, and reshaping the future of medicine.
What is AI in Drug Discovery?
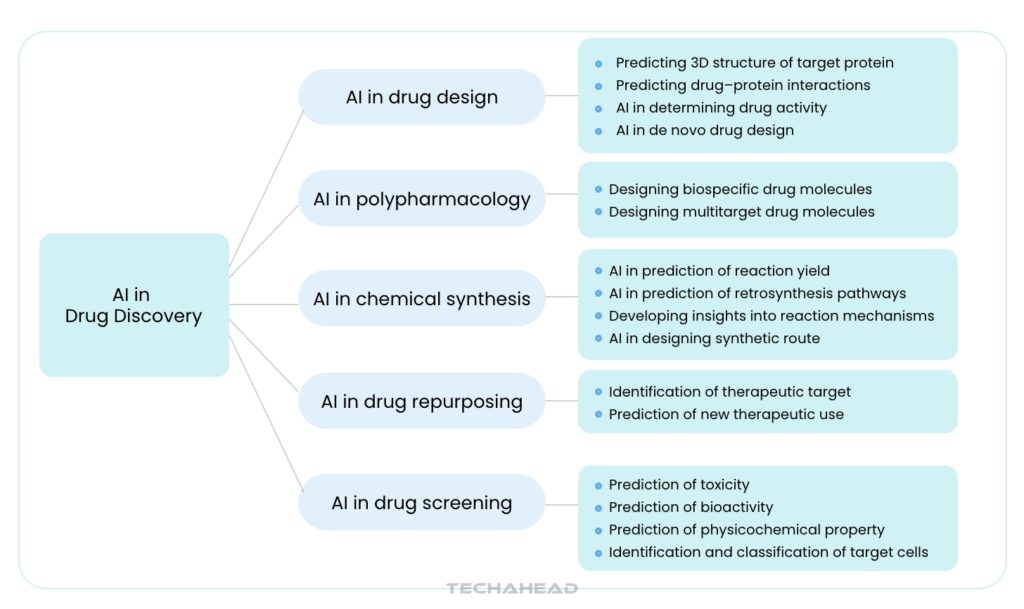
Artificial intelligence is playing an amazing role in changing drug discovery for the healthcare sector. It has changed the medical approach that tailors treatments to an individual’s genetic makeup, lifestyle, and biological characteristics.
The traditional way of drug discovery is a lengthy, costly, and uncertain journey. It often involves the synthesis of thousands of compounds, out of which a small fraction eventually proves effective in clinical trials. This inefficiency not only inflates costs but also slows down the delivery of life-saving therapies.
With the integration of AI, the landscape of drug discovery is undergoing a dramatic digital transformation. Advanced AI algorithms can predict a drug’s safety, efficacy, and mechanism of action well before it reaches the clinical trial stage.
For instance, machine learning models can analyze molecular structure and biological data to anticipate how a drug will interact with its target, reducing reliance on time-consuming lab experiments.
Moreover, AI can help identify the most promising drug targets, specific proteins, or genes associated with disease. By analyzing large-scale genomic, proteomic, and metabolomic datasets. This kind of data-driven precision enables researchers to focus on drug candidates with the highest probability of success, streamlining early-stage discovery.
How Does AI in Drug Discovery Work?
Integrating Generative AI (GenAI) into the drug discovery pipeline is revolutionizing how pharmaceutical research is conducted. Unlike traditional methods that are often slow, costly, and fragmented, GenAI leverages the power of large language models (LLMs) and enterprise-specific knowledge to streamline every stage of the R&D lifecycle.
This intelligent framework enables faster decision-making, fuels innovation, enhances precision, and ultimately shortens the time to bring life-saving drugs to market.
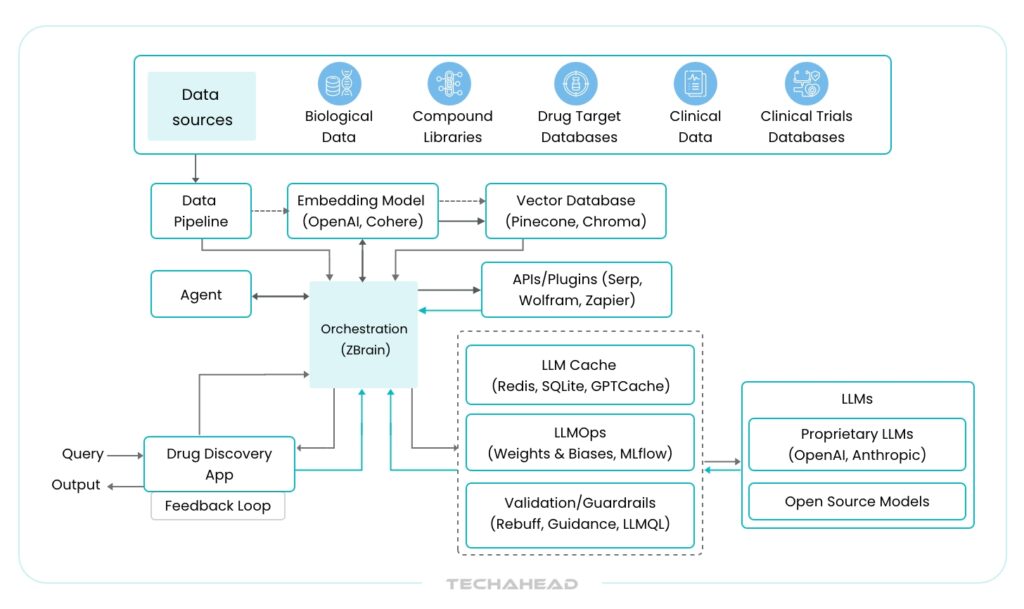
Let’s break down the core components and how they work together in a GenAI-powered drug discovery ecosystem:
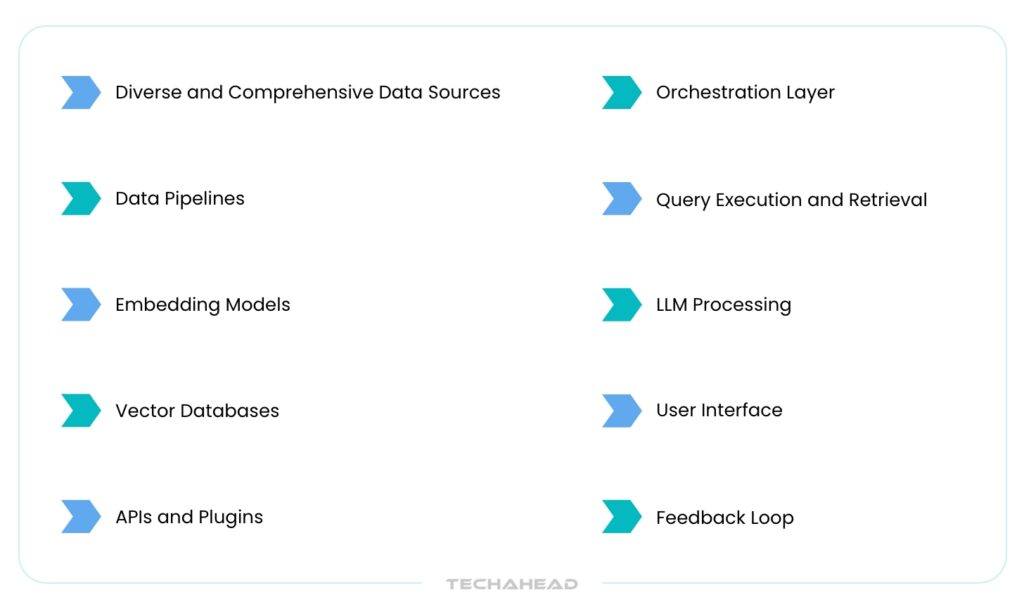
Diverse and Comprehensive Data Sources
The first step involves aggregating massive volumes of scientific and clinical data. These datasets are crucial for training the AI to understand complex biomedical relationships.
- Biological Data: Includes genomic, proteomic, and metabolomic datasets. These provide a detailed map of genetic variations, protein interactions, and biochemical changes related to disease pathways.
- Compound Libraries: Rich databases of chemical structures—both synthetic and natural—that serve as the foundation for virtual screening.
- Structure-Activity Relationship (SAR) Data: This reveals how different chemical structures impact biological activity, helping researchers refine drug candidates with better therapeutic potential.
- Drug-Target databases: Repositories that contain validated drug targets, including their biological relevance and known pharmacological interactions.
- Adverse Drug Reaction (ADR) Records: Information on previously observed side effects, which helps preempt safety concerns.
- Clinical and Trial Data: Includes patient demographics, diagnostic results, treatment outcomes, and clinical trial results—essential for modeling real-world responses.
- Scientific Literature: A vast archive of peer-reviewed papers, patents, and conference material that brings in the latest research findings and hypotheses.
Data Pipelines
Once collected, the raw data flows through automated data pipelines. These pipelines perform essential tasks such as:
- Data ingestion from multiple formats and platforms
- Cleaning to remove noise, inconsistencies, and duplicates
- Transformation, including filtering, aggregation, and normalization
- Structuring into machine-readable formats for deeper analysis
This foundational step ensures data consistency and quality across the pipeline.
Embedding Models
The refined textual data is passed through embedding models, which convert complex text into numerical vectors, mathematical representations of meaning. This vectorization process is crucial for enabling AI systems to interpret scientific concepts contextually.
Popular embedding models include those by OpenAI, Cohere, and Google, which are fine-tuned for domain-specific tasks in biomedical research.
Vector Databases
These vectors are stored in high-performance vector databases like Pinecone, Weaviate, or PGVector, which support fast similarity searches. This means the system can instantly retrieve relevant data or insights, even when searching through millions or billions of embeddings.
APIs and Plugins
Third-party APIs and plugins integrate external tools and data services into the architecture. This allows the platform to perform additional tasks like real-time computations, scientific querying, or pulling insights from external databases.
Orchestration Layer
At the heart of GenAI architecture is the orchestration layer, a control center that manages tasks and coordinates between various modules.
This layer ensures that each query is processed efficiently and contextually, yielding relevant, high-value responses.
Query Execution and Retrieval
Once a researcher submits a query about the toxicity profile of a compound or its efficacy against a particular condition, the system begins its execution process. The orchestration layer routes the query, pulls relevant information, and prepares the input for the LLM.
LLM Processing
The large language model, whether from OpenAI, Anthropic, or another provider, receives the structured input and generates an output based on the query. This response can include:
- Summarized research findings
- Novel compound suggestions
- Risk assessments
- Hypotheses on mechanisms of action
- Recommendations for experimental design
User Interface
The results are then presented via a drug discovery platform interface that consolidates insights into an intuitive, researcher-friendly dashboard. This serves as the central hub for analysis, collaboration, and decision-making.
Feedback Loop
User feedback is integral to refining the system. Whether correcting an LLM output or refining a prediction, every interaction improves model accuracy and relevance over time. This iterative loop ensures sustained performance growth.
Key Challenges in Traditional Drug Discovery Processes
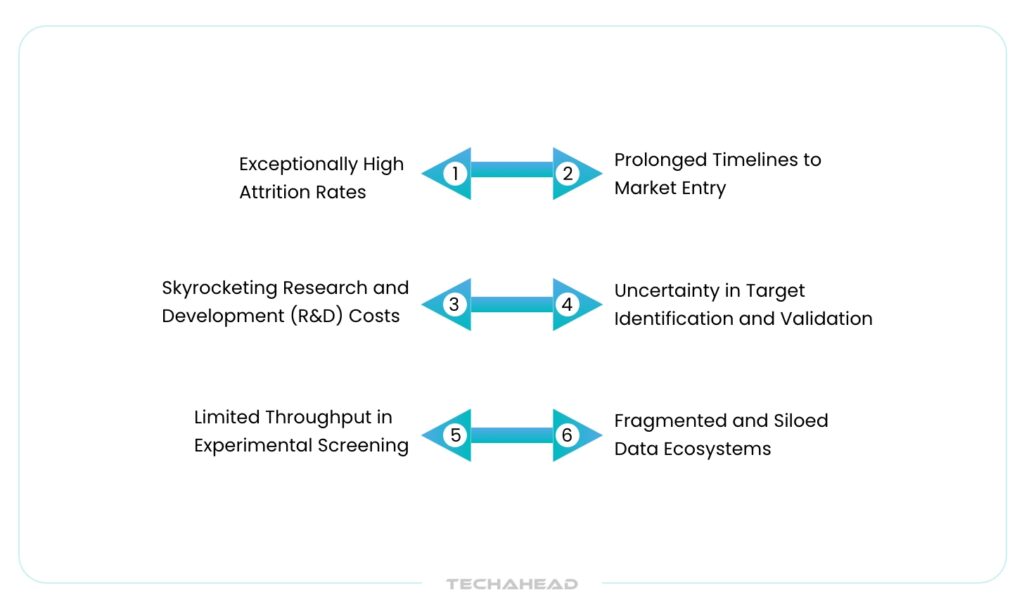
Exceptionally High Attrition Rates
Drug discovery is marked by a staggering failure rate, with nearly 90% of drug candidates failing during clinical trials, as highlighted by Nature Reviews Drug Discovery. This challenge is even more pronounced in complex therapeutic areas like Alzheimer’s disease, where an overwhelming 99.6% of clinical trials between 2002 and 2012 did not succeed.
Such significantly inflates the overall cost and time invested in development. Ultimately, these failures stem from a combination of poor target selection, unforeseen toxicities, and limited predictive power of preclinical models.
Prolonged Timelines to Market Entry
The traditional drug development pipeline is notoriously slow, with the U.S. Food and Drug Administration (FDA) estimating an average timeline of 12 years from initial discovery to final market approval. The preclinical phase alone, comprising laboratory and animal testing, consumes nearly 6.5 years, according to PhRMA.
Moreover, early-stage activities such as target identification and validation can span up to 2-3 years. Each phase involves rigorous experimentation and multiple cycles of hypothesis testing and refinement, adding layers of complexity and delay to the overall process.
Skyrocketing Research and Development (R&D) Costs
Bringing a new drug to market is not only time-consuming but also financially burdensome. The Tufts Center for the Study of Drug Development reports that the average cost to develop a single successful drug now exceeds $2.6 billion.
These costs account for both direct R&D expenses and the financial burden of failed drug candidates. Since only a small percentage of compounds eventually receive regulatory approval, the cost of failures is distributed across the few successful drugs, driving prices even higher.
Limited Throughput in Experimental Screening
Despite technological advancements, physical laboratory testing still imposes severe limitations on how many compounds can be experimentally evaluated. Traditional high-throughput screening (HTS) systems can process approximately 10,000 compounds daily, a figure that pales in comparison to the billions of possible molecular combinations that could be potential drug candidates. As a result, many promising compounds remain unexplored, and opportunities for novel therapies are missed.
Fragmented and Siloed Data Ecosystems
The volume of biomedical data has grown exponentially, yet the integration and synthesis of this data remain a major challenge. Researchers often struggle to unify diverse datasets from genomics, proteomics, electronic health records, clinical trials, and scientific publications. This fragmentation leads to inefficiencies and missed connections, preventing scientists from uncovering hidden patterns and meaningful biological insights that could accelerate drug development.
Uncertainty in Target Identification and Validation
One of the earliest and most critical steps in drug discovery is identifying a suitable biological target, such as a gene or protein associated with a disease. However, this process often relies on limited biological understanding and assumptions, making it more of an educated guess than a precise science. Even when a target appears promising in early studies, it may later prove ineffective or unsafe in clinical trials due to unexpected side effects or biological complexities that were not apparent in earlier stages.
Application of AI in Drug Discovery
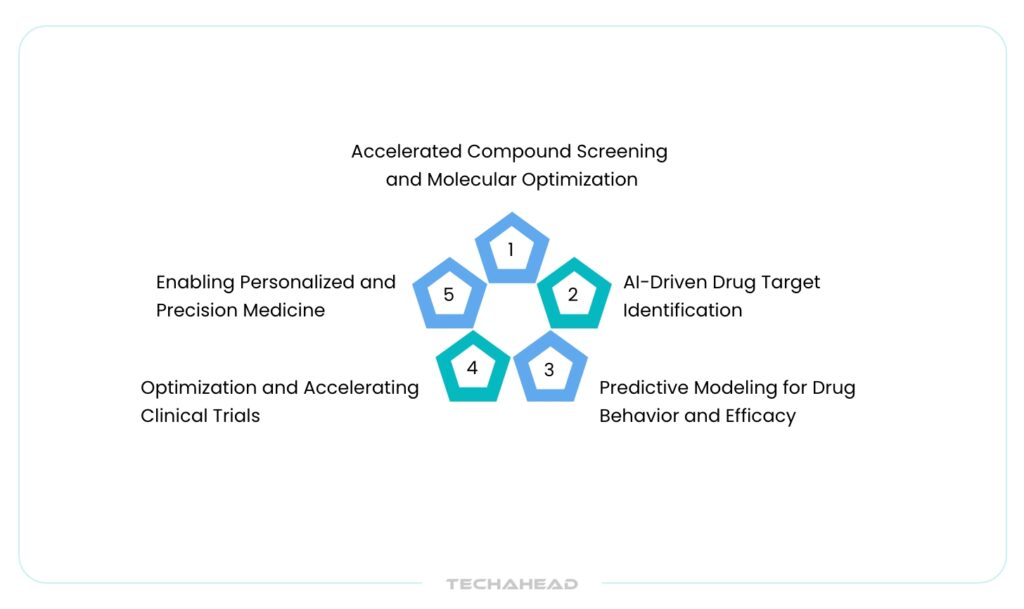
AI-Driven Drug Target Identification
One of the most critical and challenging steps in drug discovery is identifying suitable biological targets, typically genes, proteins, or molecular pathways associated with a specific disease. AI plays a transformative role here by rapidly analyzing vast, complex biological datasets to pinpoint potential druggable targets with high precision.
AI integrates genomic data, protein structural information, and disease pathway mappings to identify points of therapeutic intervention. Machine learning algorithms, especially deep learning models, can detect subtle patterns in protein-protein interactions and disease networks that might be missed by traditional methods.
These systems can also simulate how proteins behave under different conditions, predict conformational changes, and prioritize the most relevant targets for validation. AI helps decode disease mechanisms at a molecular level.
By using network-based analysis, AI can map intricate disease pathways and uncover key molecular drivers responsible for disease onset and progression. It also enables the segmentation of patient populations into disease subtypes by analyzing clinical data, thus improving the precision of target selection and supporting the development of more tailored therapies.
Accelerated Compound Screening and Molecular Optimization
Once a drug target is identified, the next step involves finding compounds that can effectively interact with it. Traditionally, this process requires screening hundreds of thousands of molecules, which is both time-consuming and expensive.
However, AI is changing with this step through virtual screening, simulating chemical interactions digitally before any wet-lab testing begins.
AI models can process massive chemical libraries and identify promising candidates in a fraction of the time it takes traditional high-throughput screening methods. Furthermore, generative AI models such as GANs (Generative Adversarial Networks) or variational structures are optimized for specific properties like target affinity, stability, and solubility.
Machine learning models also analyze structure-activity relationships (SAR) to understand how changes in molecular structure impact biological activity. Additionally, AI evaluates compound safety by predicting toxicity risks, side effects, and metabolic stability, ensuring that only the most viable candidates proceed to experimental testing.
Predictive Modeling for Drug Behavior and Efficacy
Before a compound can enter human trials, it’s essential to predict how it will behave inside the body. This is where AI steps in with sophisticated predictive modeling tools that simulate pharmacokinetics (PK) and pharmacodynamics (PD), the processes by which a drug is absorbed, distributed, metabolized, and excreted, and how it affects the body.
AI can forecast how quickly a drug is absorbed, how it travels through tissues, and how long it remains active in the bloodstream. These insights inform dosing strategies and help in minimizing adverse reactions.
Moreover, AI models can simulate drug interactions, which is important for patients on multiple medications. By analyzing how different compounds influence the same pathways or organs, AI can help researchers design safer drug combinations and avoid harmful side effects. This significantly reduces the risk of late-stage clinical trial failure due to unforeseen interactions.
Optimization and Accelerating Clinical Trials
Clinical trials are not only expensive but also prone to delays due to poor participant matching, slow recruitment, and unexpected side effects. AI helps address these issues by improving both patient selection and trial monitoring.
Using advanced analytics, AI can identify suitable participants based on a wide range of criteria, including genetic markers, medical history, lifestyle data, and predicted drug response. This ensures that the right patients are included in the right trials, improving the odds of success. AI also aids in stratifying patients into subgroups for precision trials, which increases the likelihood of detecting meaningful treatment effects.
During the trial, AI continuously monitors incoming patient data, flagging any early signs of adverse events or lack of efficacy. Real-time analysis of electronic health records (EHRs), wearable device data, and lab results allows researchers to make informed decisions about trial adjustments or even early termination. Additionally, AI automates the tedious process of analyzing clinical trial documentation, saving both time and resources.
Enabling Personalized and Precision Medicine
AI is paving the way for personalized medicine, an approach that customizes treatment plans to each individual’s unique genetic and clinical profile. This shift reduces the traditional trial-and-error prescribing method and significantly improves treatment outcomes.
By analyzing a patient’s genetic data, AI can predict how that individual will metabolize certain drugs, identify potential adverse reactions, and recommend optimal drug types and doses. AI models also incorporate other variables such as family history, lifestyle, and environmental exposure to create a comprehensive treatment plan tailored specifically to the patient.
Furthermore, AI supports adaptive treatment strategies by monitoring real-time patient responses. Based on this feedback, therapy plans can be adjusted dynamically to ensure maximum effectiveness with minimal side effects. In cases of complex conditions, AI can even recommend personalized drug combinations and dosing regimens that are fine-tuned to the patient’s biology.
Advantages of Leveraging AI in Drug Discovery
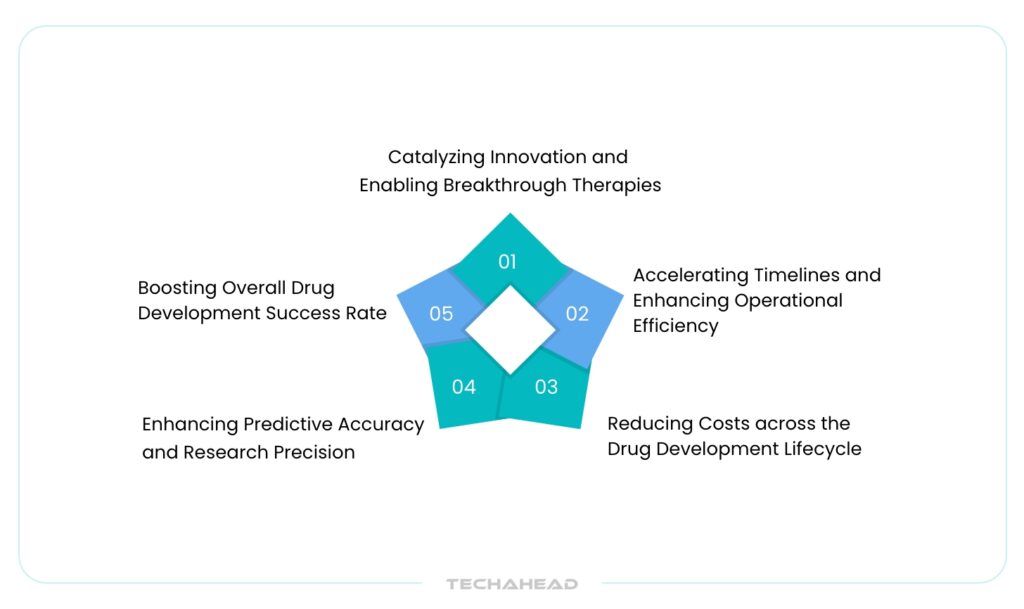
Catalyzing Innovation and Enabling Breakthrough Therapies
AI is reshaping the landscape of pharmaceutical research by acting as a catalyst for innovation. It allows scientists to explore previously unreachable therapeutic territories, driving the creation of next-generation treatments.
- Empowering precision medicine: AI helps tailor treatments to each patient’s unique genetic blueprint. By analyzing vast genomic datasets, AI uncovers how specific genes influence drug response, thereby enabling the development of highly personalized therapies. This ensures not only greater efficacy but also minimizes adverse reactions.
- Identifying novel therapeutic targets: Traditional methods often miss subtle biological signals, but AI excels at detecting these. By analyzing complex biological networks, AI reveals hidden or non-obvious targets, opening the door to innovative treatments, especially for diseases with limited existing therapies.
- Designing novel chemical entities: Through the use of generative AI models, researchers can design entirely new compounds from scratch. These AI-generated molecules are customized with specific pharmacological properties, significantly increasing the odds of discovering first-in-class drugs.
- Unraveling complex disease mechanisms: Many conditions like cancer, neurodegenerative disorders, and autoimmune diseases involve multiple pathways and factors. AI enables the detailed study of these multifactorial diseases by mapping out intricate interactions across genetic, proteomic, and metabolic layers, ultimately leading to better-targeted interventions.
Accelerating Timelines and Enhancing Operational Efficiency
Speed is a critical advantage in the pharmaceutical industry, where delays can cost lives and billions of dollars. AI dramatically improves operational efficiency by optimizing and automating core research tasks.
- Rapid data processing and analysis: AI tools can analyze terabytes of biological and chemical data in minutes, work that might take traditional teams weeks or months. This capability allows researchers to quickly identify promising molecules or biological pathways for further investigation.
- Automation of repetitive tasks: Tasks such as compound screening, data labeling, and documentation review are time-intensive and error-prone when done manually. AI automates these functions, improving productivity and freeing up scientists to focus on high-impact, strategic problems.
- Expedited target validation: AI models can simulate biological interactions and validate potential targets early in the process. By filtering out weak candidates, AI helps teams concentrate their efforts on the most promising leads, effectively shortening development cycles.
- Facilitating collaborative research: Centralized data platforms powered by AI enable seamless collaboration across R&D teams, departments, and even organizations. This ensures better alignment, faster decision-making, and real-time sharing of insights.
Reducing Costs across the Drug Development Lifecycle
Drug development is notoriously expensive, but AI introduces numerous cost-saving mechanisms without compromising quality or outcomes.
- Lower R&D expenditure: By automating data analysis and eliminating unnecessary lab experiments, AI reduces dependence on costly wet-lab operations. This translates to substantial savings on materials, manpower, and infrastructure.
- Minimizing risk of failures: Predictive algorithms can identify high-risk compounds early, preventing them from advancing to expensive late-stage testing. This proactive screening minimizes sunk costs and redirects investment to more viable candidates.
- Smart resource allocation: AI ranks research projects based on data-driven insights, helping organizations allocate their time, budget, and manpower to areas with the highest success potential.
- Faster market readiness: Accelerated discovery and development pipelines mean drugs reach the market sooner. This shortens the return-on-investment period and allows pharmaceutical companies to recoup development costs more quickly.
Enhancing Predictive Accuracy and Research Precision
Precision and reliability are vital in drug discovery, and AI strengthens both through its powerful analytical and predictive capabilities.
- High-precision discovery: AI algorithms can differentiate between viable and non-viable biological targets with remarkable accuracy. This leads to higher success rates in early-stage drug development and lowers the chance of failures in later stages.
- Reliable efficiency forecasting: Machine learning models use vast historical datasets to predict how effective a compound will be in treating a specific disease. These insights guide scientists in selecting the most promising molecules for advancement.
- Early toxicity detection: AI tools evaluate the chemical structure of compounds to forecast potential toxicity risks before they ever reach animal or human trials. Early safety insights prevent costly recalls and protect patients.
- Data-driven strategy formation: AI systems turn raw data into actionable insights, enabling research teams to make informed decisions based on solid scientific evidence rather than intuition or trial-and-error methods.
Boosting Overall Drug Development Success Rates
Ultimately, AI contributes to higher success rates across the entire drug development continuum, from initial discovery through to regulatory approval.
- Improved candidate selection: By continuously learning from past research data, AI helps in identifying high-quality candidates that have better chances of passing clinical trials and making it to market.
- Smarter clinical trial design: AI streamlines the design of clinical trials by selecting optimal participant groups and predicting potential trial outcomes. This leads to better data quality and higher success rates in demonstrating drug efficacy.
- Continuous model refinement: AI systems evolve over time. With every new data point, their predictive accuracy improves, making each subsequent drug discovery project more efficient and effective than the last.
- Proactive risk management: Through early detection of red flags, be it toxicity, lack of efficacy, or poor bioavailability. AI enables companies to course-correct early, thereby reducing regulatory hurdles and increasing the probability of approval.
Conclusion
The integration of AI into drug discovery marks a transformative shift in how modern pharmaceuticals are researched and developed. Unlike traditional methods, which often rely on time-consuming trial-and-error processes, generative AI enables a more data-driven, predictive, and efficient approach. It plays a critical role in designing novel molecular structures, identifying viable biological targets, and optimizing compound properties early in the pipeline, tasks that are typically resource-intensive and complex.
By analyzing vast and diverse datasets, ranging from genomic and proteomic data to chemical libraries and clinical outcomes, AI helps uncover hidden relationships and therapeutic opportunities that may otherwise go unnoticed. This expanded analytical scope supports better decision-making, improves the quality of drug candidates, and accelerates the path from discovery to development.
As the pharmaceutical industry continues to embrace these advanced AI capabilities, generative AI is emerging as a central force in addressing unmet medical needs and driving the development of safer, more effective treatments.

FAQs
AI isn’t meant to fully replace traditional drug development; rather, it enhances and accelerates the process. By analyzing large datasets, predicting molecular behaviors, and identifying promising drug candidates, AI can significantly streamline early research. However, real-world testing and clinical trials remain essential to ensure safety and effectiveness.
AI brings transformative advantages to drug discovery by reducing development costs and accelerating timelines. This increased efficiency can make medications more affordable and accessible while also opening doors to treatments for diseases that currently lack effective cures.
Artificial Intelligence accelerates drug development by combining vast data sources, computational strength, and intelligent algorithms. This integration streamlines research, improves the accuracy of predictions, increases success rates, and significantly shortens the time required to bring new therapies to market.
Beyond discovering entirely new drugs, AI is also used to repurpose existing, approved medications. By analyzing large biomedical datasets, AI can uncover new therapeutic uses for known compounds, helping researchers fast-track the development of effective treatments for a variety of conditions.
AI plays a crucial role in advancing drug research by predicting how chemical compounds will behave in the body based on their molecular structures. This predictive capability enhances the efficiency and effectiveness of developing new drugs, ultimately improving outcomes in both drug discovery and healthcare.